The QM Solution for your Pharmacy
The QM Solution for your Pharmacy









By digitalizing our QM documentation with roXtra, we can optimally prepare audits for our pharmacies.
Philipp Wälde, Owner BLESS YOU Pharmacies
success stories
Find out first-hand how users use roXtra to design their quality management systems (QMS) efficiently and in compliance with regulations. Whether ISO 9001, ISO 13485 or the QM guidelines of the German Federal Chamber of Pharmacists (BAK) - roXtra offers solutions that are specially tailored to the requirements of pharmacies.
In our blog, you can find out more about certification and re-certification, the implementation of quality management in pharmacies and practical application examples from the industry.

Special requirements for QM software
for decentralized project processes
Asklepios clinics
Get advice now
Get to know roXtra in a non-binding and free online presentation.
What is a quality management system in a pharmacy?
Quality management in pharmacies (QM) encompasses all measures that ensure consistently high quality in the manufacture, testing and dispensing of medicines. This includes structured processes, complete documentation and regular audits in order to meet legal requirements and customer demands. Powerful QM software supports pharmacies in document control, process management, audit planning and risk management and facilitates the implementation of continuous improvement measures.
Modern software solutions for quality management in pharmacies automate processes and ensure greater transparency and efficiency. This minimizes sources of error, optimizes work processes and makes it easier to comply with legal requirements.
What activities does quality management in pharmacies involve?
Quality management in pharmacies involves numerous tasks that serve both to comply with legal requirements and to optimize internal processes. This includes document control in order to keep all relevant work instructions, process descriptions and protocols up to date and audit-proof. Process management also plays a central role by defining standardized processes and ensuring compliance with them. Efficient QM software for pharmacies helps to manage these tasks in a structured and digital way.
Other important activities in quality management for pharmacies are audit management, which is used to plan and document internal and external audits. Risk management enables potential risks to be identified and minimized at an early stage. Systematic action management allows necessary improvements to be implemented quickly. Digital QM software solutions ensure efficient implementation of all QM processes and help pharmacies to permanently meet the high quality and safety requirements.
Why is quality management important in pharmacies?
Quality management in pharmacies is essential to ensure a safe and reliable supply of medicines. Standardized processes minimize errors, increase patient safety and comply with legal requirements. In addition, structured quality management helps to increase efficiency, improves customer satisfaction and promotes the continuous development of pharmacy processes. In an increasingly regulated and competitive environment, it ensures the long-term success and trustworthiness of the pharmacy.
How can a pharmacy manage its QM documents efficiently?
A pharmacy can manage its QM documents efficiently by using a digital document management solution that enables central storage, versioning and access control. Clear responsibilities and regular reviews ensure that all documents are up-to-date and legally compliant. In addition, a structured folder hierarchy or specialized QM software makes it easier to find and process relevant documents quickly. Training for employees and the integration of automated workflows also help to minimize administrative work and ensure compliance with quality standards.
How can a pharmacy carry out internal audits efficiently?
A pharmacy can carry out internal audits efficiently by using specialized audit software. This enables structured planning, execution and documentation of audits. Digital checklists and automated reminders ensure a complete review of relevant processes, while integrated reporting and analysis functions quickly identify weaknesses. The software also makes it easier to track corrective measures and ensures transparent communication within the team. The central storage of all audit data reduces administrative effort and supports compliance with legal requirements in the long term.
One software, many application areas.
Pharmacy quality management: Effective document control with roXtra
Quality and safety are essential in pharmacies in order to comply with legal requirements and ensure reliable patient care. Regulatory requirements such as the German Pharmacy Operating Regulations (ApBetrO) or Good Manufacturing Practice (GMP) require structured quality management (QM).
The roXtra document control module makes managing your QM documentation - from procedural instructions and hygiene regulations to test protocols - much easier. Powerful software for quality management in pharmacies accompanies the entire document life cycle and supports the compliant implementation of regulations.
Your advantages with roXtra in the area of document control:
- Individual approval workflows: Customizable to your processes and standard requirements.
- Audit-proof versioning: automatic storage and archiving.
- Compliant document management: For all guidelines, e.g. Pharmacy Operating Regulations (ApBetrO) or GMP.
- Rely on a future-proof software solution to make quality management in your pharmacy efficient, compliant and transparent.
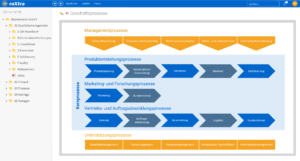
Process management for pharmacies: efficient and digital
Optimize internal processes with a digital solution for quality management in your pharmacy. The roXtra Processes module makes it easy to model and control processes - from formulation production to hygiene checks and complaints management.
With an intuitive graphical interface, you can define rules, responsibilities and tasks for all relevant processes. This ensures that all quality management specifications are implemented efficiently in your pharmacy and that legal requirements are met.
With the Flowchart Designer, you can create simple models such as flowcharts, organization charts or mind maps in no time at all and make your processes easy to understand for everyone involved.
With the Flowchart Designer, you can create simple models such as flowcharts, organization charts or mind maps in no time at all and make your processes easy to understand for everyone involved.
Advantages of process management with roXtra:
- Transparency and clarity: Visualization of complex processes such as recipe processes or test procedures.
- Continuous improvement (CIP): Optimize processes and make them sustainably efficient.
- User-friendliness: Intuitive design and fast implementation of your requirements.
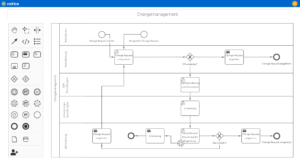
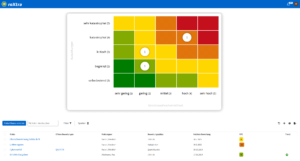
Risk management for pharmacies with roXtra risks
With roXtra Risks, you minimize the effort involved in risk management. Identify, analyze and manage potential risks centrally to ensure safety and quality in your pharmacy. The QM software supports regulatory requirements such as the German Pharmacy Operating Regulations (ApBetrO), GMP and ISO 9001 and facilitates the implementation of effective quality management in pharmacies.
Effective risk management for maximum safety
Regulatory requirements demand structured risk management in order to identify risks such as incorrect dispensing of medicines or hygiene deficiencies at an early stage. The QM software from roXtra helps you to systematically assess risks, initiate measures and continuously optimize your QM processes.
Effective audit management for pharmacies with roXtra audits
Audits are a central component of quality management in pharmacies in order to check processes, uncover non-conformities and promote continuous improvement.
With the roXtra Audits module, you can plan, document and manage internal and external audits efficiently. The QM software helps you to manage audit processes transparently, derive measures directly and ensure traceability.
Your advantages with roXtra Audits:
- Efficiency and transparency: Document and initiate immediate, corrective and preventive measures directly in the context of the audit.
- Systematic implementation: Access your questionnaire from any location - even offline.
- Optimized planning: Maintain an overview of past, current and planned audits.
Measure management for pharmacies with roXtra measures
Structured action management in pharmacies makes it possible to identify deviations at an early stage, minimize risks and comply with legal requirements such as ISO 9001 and the German Pharmacy Operating Regulations (ApBetrO). With roXtra measures, immediate, corrective and preventive actions (CAPA) can be planned, controlled and tracked in a targeted manner.
The roXtra QM software ensures complete documentation and transparent tracking of all measures. This not only supports quality management in your pharmacy, but also facilitates continuous improvement and compliance with regulatory requirements.
Your advantages with roXtra measures:
- Central management: Clearly record and control all measures.
- Automatic effectiveness check: Sustainable implementation through planned checks.
- Simple evaluation: Filter lists of measures and export them as Excel, Word or PDF.
- Cross-module: Linking for consistent documentation.
Support for certifications and QM in pharmacies
Our software solutions make your daily operations as well as the certification and re-certification of your quality management system (QMS) easier. With roXtra, you can implement a wide range of standards and regulations efficiently and in compliance with the rules - from ISO 9001 to ISO 27001.
Quality management in pharmacies
- Quality certificate of the Chamber of Pharmacists
- QM statutes of the Chamber of Pharmacists
- QM guidelines of the Federal Chamber of Pharmacists (BAK)
- Pharmacy operating regulations - § 2a Quality management system (ApBetrO)
- BAK quality seal
Risk management and security
- DIN EN ISO 31000: Guidelines for risk management
- ONR 49000 / ONR 49001: Austrian risk management standards
- IEC 80001: Risk management for networked medical technology
- KonTraG and Section 91 (2) AktG: Establishment of a risk management system (RMS) in the corporate sector and for listed companies
Quality management in general
- DIN EN ISO 9000 series of standards: Quality management standards
- DIN EN ISO 9001: Quality management systems
- ISO 27001: Information security management system (ISMS) and IT security
roXtra supports you with the following solutions:
We will show you roXtra in a free and non-binding online presentation.






