The software for quality management in medical practices
The QM software for your medical practice
success stories
Find out first-hand how customers use roXtra to make their quality management in medical practices efficient, compliant and future-proof. Whether ISO 9001, hygiene and risk management or structured document control - roXtra offers customized solutions for the specific requirements of everyday practice.
Our blog provides you with valuable insights into certification and re-certification, the practical implementation of quality management for medical practices as well as best practices and application examples from the industry. Whether QM for dental practices, GP, specialist or veterinary practices - discover how software can optimize and sustainably improve your quality management.

Quality management in the medical practice - requirements and advantages
Marion Meyer
Get advice now
Get to know roXtra in a non-binding and free online presentation.
Efficient quality management in practices
Well-organized quality management in medical practices makes a decisive contribution to patient safety and the optimization of internal processes. With roXtra document control, you can manage your QM documentation efficiently and in compliance with regulations - from hygiene concepts and procedural instructions to emergency plans and work instructions. Our software for medical practices helps you to record, update and centrally provide all relevant documents in a structured manner.
Automatic versioning, audit-proof archiving and customizable workflows allow you to review and approve new or revised documents in a clear and transparent manner. During an audit, all important QM documents, including metadata such as resubmission date or revision status, are quickly available.
With roXtra, you can optimize quality management in medical practices, avoid unnecessary sources of error and ensure seamless documentation. Whether QM for dental practices, GP, specialist or veterinary practices - efficient QM software makes everyday practice life easier and supports compliance with legal requirements.
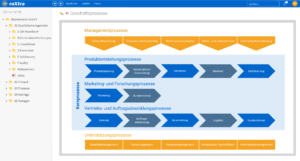
Digitally control and optimize processes in practices
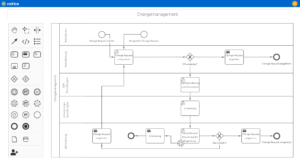
Clear and structured processes are the basis for efficient quality management in medical practices. With roXtra Processes, you can digitize and automate internal practice workflows - for example for maintenance schedules or vacation requests. Thanks to the graphical modeling (BPMN 2.0), responsibilities, approval processes and workflows can be individually adapted.
The Flowchart Designer enables quick visualization of flowcharts, organization charts and mind maps, so that workflows are presented in a structured and understandable way.
The Flowchart Designer enables quick visualization of flowcharts, organization charts and mind maps, so that workflows are presented in a structured and understandable way.
With roXtra processes, you can optimize your quality management in medical practices, automate recurring processes and ensure audit-proof, standard-compliant documentation.
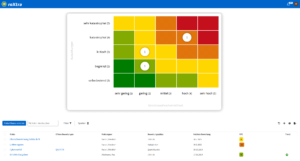
Structured risk management
Well thought-out quality management in medical practices also includes effective risk management to ensure the safety of patients and practice processes. With roXtra Risks, you can identify, evaluate and manage potential risks centrally, reduce administrative effort and ensure systematic risk analysis. The software supports common standards and regulations such as ISO 9001, ISO 31000 and ISO 27001 as well as industry-specific guidelines, including Rili-BÄK.
Regular risk assessments are essential in order to identify and minimize risks at an early stage - be it in hygiene, medical technology, patient care or data protection. Structured quality management for medical practices helps to avoid operational risks and ensure safe, standard-compliant practice management.
With roXtra Risks, you can optimize your QM for dental practices, general practitioners, specialists and veterinary practices and ensure seamless documentation. Powerful software for medical practices helps you to manage risks in a targeted manner and provide an audit-proof, transparent solution for audits and certifications.
Professionally manage audits in practices
Regular audits are essential to ensure compliance with standards and legal requirements and to ensure structured quality management in medical practices. With roXtra Audits, you can optimize your audit management and carry out internal and external audits (e.g. in accordance with ISO 19011) efficiently and transparently.
Our software for medical practices gives you location-independent access to audit questionnaires - even offline. This means you always have an overview of past, current and planned audits and can identify non-conformities and potential for improvement at an early stage. Defined immediate, corrective and preventive measures can be documented and implemented directly in the audit context.
Whether QM for dental practices, GP, specialist or veterinary practices - with roXtra Audits you can make your quality management compliant, transparent and efficient.
Action management for practices
Structured quality management in medical practices requires efficient action management in order to eliminate deviations, minimize risks and meet legal and normative requirements such as ISO 9001. With roXtra Measures, you can manage immediate, corrective and preventive actions (CAPA) systematically, digitally and transparently.
roXtra Measures enables seamless documentation and structured monitoring of all measures so that you can implement continuous improvements in a targeted manner.
Your advantages with roXtra measures:
- Central management: Clearly record and control all measures.
- Automatic effectiveness check: Sustainable implementation through planned checks.
- Simple evaluation: Filter lists of measures and export them as Excel, Word or PDF.
- Cross-module: Linking for consistent documentation.
Support for quality management and certifications for practices
Our software solutions for (medical) practices support you in your day-to-day work as well as in the certification and re-certification of your quality management system. With roXtra, you can efficiently implement a wide range of standards and regulations and make quality management in your practice safe, transparent and compliant.
Quality management standards
- DIN EN 15224 Quality management systems for the health care sector
- ISO 7101 Quality management systems in healthcare - Requirements
- DIN EN ISO 9001 - Requirements for quality management systems
- DIN EN ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes
Risk management standards
- ISO 22367 - Risk management for error reduction in medical laboratories
- ISO 31000 - General guidelines on risk management
- ONR 49001 - Austrian standard for systematic risk management
- IEC 80001 standard for risk management in networked medical technology
- Section 91 (2) AktG - Establishment of a risk management system (RMS) for listed companies
Information security management
- ISO 27001 - Information Security Management System (ISMS) / IT Security
Specific guidelines and laws
- QEP (Quality and Development in Practices) of the Associations of Statutory Health Insurance Physicians (NASHIP) together with the Federal Association of Statutory Health Insurance Physicians
- KTQ Certification: Cooperation for Transparency and Quality in the Healthcare Sector
- European Practice Assessment (EPA)
- Claims of the Federal Joint Committee (G-BA), the German Accreditation Body GmbH (DAkkS), the German Nursing Council (Deutscher Pflegerat e.V. - DPR) as well as the Initiative Quality Medicine (IQM)
- KonTraG & Section 91 (2) AktG - Regulations on risk control in companies
One software, many application areas.
What is part of quality management in the medical practice?
Quality management in medical practices includes structured processes to ensure high treatment quality and patient safety. This includes document control, process optimization as well as audit and risk management. Modern software for medical practices supports implementation, e.g. through digital documentation and process automation. Effective quality management for medical practices helps to comply with standards such as ISO 9001 and improves processes in GP and specialist practices as well as in QM for dental practices.
Are medical practices obliged to use QM?
Yes, many medical practices are obliged to introduce quality management in the medical practice. This applies to GP and specialist practices as well as to QM for dental practices. Specialized software facilitates implementation by managing documentation, processes and audits digitally. Effective quality management for practices not only helps to comply with legal requirements, but also improves processes and patient safety.
How can digital QM software help to make practice processes more efficient?
Software optimizes quality management in medical practices by automating workflows, managing documents centrally and making processes more transparent. Through structured workflows and audit-proof archiving, it facilitates the implementation of quality management and ensures efficient, standard-compliant practice organization. Whether appointment management, hygiene management or audit processes - a modern solution supports QM for dental practices, general practices and specialist practices.
roXtra supports you with the following solutions:
We will show you roXtra in a free and non-binding online presentation.







