The QM system for medical devices:
Your efficient software solution
QM system for medical devices: Your software solution
success stories
Find out first-hand how medical device manufacturers use roXtra to make their quality management systems (QMS) efficient and compliant. Whether ISO 13485, MDR, FDA regulations or comprehensive risk management - roXtra offers solutions that are specially tailored to the requirements of medical technology.
In our blog, you can find out more about certification and re-certification, the implementation of quality management in medical technology and practical application examples from the industry.

Digital quality management at the laboratory service provider
TRIGA-S Scientific Solutions
Get advice now
Get to know roXtra in a non-binding and free online presentation.
What is a quality management system (QMS) for medical device manufacturers?
A quality management system (QMS) for medical device manufacturers organizes and monitors all quality-related processes to ensure compliance with legal and regulatory requirements such as ISO 13485, MDR and FDA regulations. It includes document control, risk management, process control and the performance of audits.
The aim is to ensure product quality and safety, minimize risks and implement continuous improvements. A QM system is essential in order to manufacture medical devices efficiently, secure market approvals and strengthen the trust of customers and authorities.
Do medical device manufacturers have to provide evidence of a QM system?
Yes, medical device manufacturers must provide evidence of a quality management system (QM system), as this is a prerequisite for market approval. In the EU, the MDR requires a QM system in accordance with ISO 13485, while in the USA the FDA stipulates compliance with 21 CFR Part 820. A QM system ensures the safety, efficacy and conformity of products through clearly defined processes such as document control, risk management and traceability. Proof is usually provided through certification by a notified body, which is essential for access to regulated markets.
What is the aim of a QM system according to ISO 13485?
ISO 13485 describes the requirements for a quality management system (QMS) specifically for manufacturers and suppliers of medical devices. The aim is to ensure the safety and effectiveness of medical devices by systematically controlling and monitoring all quality-relevant processes.
What are the advantages of software for the QM system?
QMS software optimizes processes, increases efficiency and ensures compliance with regulations. It automates document control, audit planning and CAPA measures, supports standards such as ISO 13485 and MDR and ensures complete traceability. Thanks to flexible workflows, systematic data evaluation and standardized processes, it reduces errors, saves costs and promotes continuous improvement (CIP). An indispensable tool for quality and compliance in medical technology.
Does QM software help me in the audit?
Yes, QM software provides you with comprehensive support in the planning, implementation and follow-up of audits. It facilitates the creation of audit plans and checklists, enables the digital recording of findings and documents non-conformities directly in the system. Automated reports and the linking of measures such as CAPA (Corrective and Preventive Actions) allow you to maintain an overview and ensure that all deviations are processed on time. The software also ensures complete traceability and helps you to efficiently meet regulatory requirements such as ISO 13485, MDR or 21 CFR Part 11. This increases the effectiveness of your audits and saves you valuable time.
One software, many application areas.
Effective document control with roXtra
In medical technology, product quality and reliability are essential. Standards such as ISO 13485 and regulations such as Good Working Practice (GxP) require an effective and structured quality management system (QMS).
The roXtra document control module makes managing your QM documentation, such as procedural instructions, safety and performance guidelines or product descriptions, much easier. The document management system (DMS) accompanies the entire life cycle of your documents and helps you to implement regulatory requirements efficiently.
Your advantages with roXtra in the area of document control:
- Individual approval workflows: Customizable to your processes and standard requirements.
- Audit-proof versioning: automatic storage and archiving.
- Rule-compliant document management: For all guidelines, e.g. ISO 13485 or GxP.
- Rely on a future-proof software solution to make your QM processes in the field of medical devices efficient, compliant and transparent.
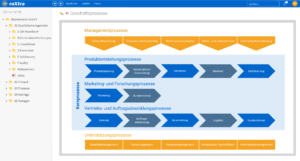
Process management for medical technology: Efficient and digital with roXtra processes
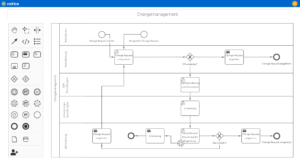
Digitize and automate your internal processes with the roXtra Processes module. Thanks to the graphical modeling according to BPMN 2.0, you can easily define and control rules, responsibilities, tasks and input fields for various processes and forms (e.g. test reports, test procedures).
roXtra Processes enables you to design your processes efficiently, digitally and in compliance with regulations - perfectly adapted to the requirements of medical technology.
With the Flowchart Designer, you can create simple models such as flowcharts, organization charts or mind maps in no time at all and make your processes easy to understand for everyone involved.
With the Flowchart Designer, you can create simple models such as flowcharts, organization charts or mind maps in no time at all and make your processes easy to understand for everyone involved.
Advantages of process management with roXtra:
- Transparency and clarity: Visualization of complex processes such as conformity assessment procedures or post-market surveillance.
- Continuous improvement (CIP): Optimize processes and make them sustainably efficient.
- User-friendliness: Intuitive design and fast implementation of your requirements.
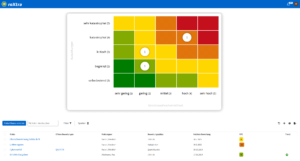
Risk management for medical devices with roXtra risks
With roXtra Risks, you can reduce your risk management workload and improve your risk analysis. Identify and manage potential risks centrally to ensure the safety and quality of your medical devices. The software supports common standards and regulations, including ISO 14971, MDR, IVDR, ISO 31000 and ISO 9001.
Effective risk management for maximum safety
Regulatory requirements such as ISO 14971 demand an effective risk management system to minimize risks. With roXtra, you can identify and evaluate risks such as incorrect operation or product faults and avoid them by taking targeted measures. This allows you to sustainably improve patient safety and meet regulatory requirements.
Effective audit management with roXtra audits
Audits are an indispensable part of your work as a quality manager and internal auditor. Systematic audit management is your most powerful tool for checking processes, uncovering non-conformities and driving improvements.
With the roXtra Audits module, you can increase the effectiveness and quality of your work. The software supports you in carrying out and managing internal audits and auditing management systems in accordance with specifications such as ISO 19011.
Whether DIN EN ISO 9001, ISO 13485, DIN EN ISO/IEC 17025 or ISO 14001 - with roXtra Audits you can easily meet the requirements of current standards. The software helps you to identify potential for improvement, systematically plan and carry out internal audits and ensure traceability.
Your advantages with roXtra Audits:
- Efficiency and transparency: Document and initiate immediate, corrective and preventive measures directly in the context of the audit.
- Systematic implementation: Access your questionnaire from any location - even offline.
- Optimized planning: Maintain an overview of past, current and planned audits.
Measures management for medical devices with roXtra measures
Effective action management with roXtra actions is crucial for eliminating deviations, minimizing risks and meeting the requirements of standards such as ISO 13485 and MDR. With a structured approach, immediate, corrective and preventive actions (CAPA) can be efficiently planned, implemented and tracked.
A digitally supported system guarantees complete documentation and transparent monitoring of all measures, ensuring continuous improvement and compliance with regulatory requirements.
Your advantages with roXtra measures:
- Central management: Clearly record and control all measures.
- Automatic effectiveness check: Sustainable implementation through planned checks.
- Simple evaluation: Filter lists of measures and export them as Excel, Word or PDF.
- Cross-module: Linking for consistent documentation.
Support for quality management and certifications in medical technology
Our software solutions make your daily operations as well as the certification and re-certification of your quality management system (QMS) easier. With roXtra, you can implement a wide range of standards and regulations efficiently and in compliance with the rules - from DIN EN ISO 13485 to ISO 27001.
Quality management in medical technology
- DIN EN ISO 13485: Quality management for medical devices
- MDR (Medical Device Regulation) and MDD (Medical Device Directive): EU regulations for medical devices
- Medical Devices Act (MPG): Legal requirements for medical devices
- FDA regulations: Quality management for medical devices in the USA
- Medical Devices Operator Ordinance (MPBetreibV): Requirements for the installation, operation and use of medical devices
- G-BA claims: Guidelines of the Federal Joint Committee
Risk management and security
- DIN EN ISO 14971 / OE 14971: Application of risk management to medical devices
- DIN EN ISO 31000: Guidelines for effective risk management
- ONR 49000 / ONR 49001: Austrian risk management standards
- IEC 80001: Risk management for networked medical technology
- KonTraG and Section 91 (2) AktG: Establishment of a risk management system (RMS) for listed companies
Quality management in general
- DIN EN ISO 9000 series of standards: Quality management standards
- DIN EN ISO 9001: Quality management systems for companies
- ISO 27001: Information security management system (ISMS) and IT security
roXtra supports you with the following solutions:
We will show you roXtra in a free and non-binding online presentation.







