Die QM-Software für Medizinische Versorgungszentren
Die QM-Software für Ihr medizinisches Versorgungszentrum

I have been working with different companies for years and have never been so permanently and sustainably well looked after.
What our customers say about roXtra
🡥 Customer survey 2024
success stories
Erfahren Sie aus erster Hand, wie Kunden roXtra nutzen, um ihr Qualitätsmanagement in Medizinischen Versorgungszentren effizient, regelkonform und zukunftssicher zu gestalten. Ob ISO 9001, Hygiene- und Risikomanagement oder die strukturierte Dokumentenlenkung – mit unserer Software für Ihr MVZ erhalten Sie maßgeschneiderte Lösungen für die speziellen Anforderungen im Arbeitsalltag eines medizinischen Versorgungszentrums.
In unserem Blog erhalten Sie wertvolle Einblicke zur Zertifizierung und Re-Zertifizierung sowie zur praktischen Umsetzung von Qualitätsmanagement für Medizinische Versorgungszentren. Entdecken Sie anhand von Best Practices und Anwendungsbeispielen, wie eine leistungsstarke Software Ihr Qualitätsmanagement optimiert und nachhaltig verbessert – für eine reibungslose Organisation und eine effiziente Patientenversorgung.

Quality management in the medical practice - requirements and advantages
Marion Meyer
Get advice now
Get to know roXtra in a non-binding and free online presentation.
Effizientes Qualitätsmanagement in medizinischen Versorgungszentren
Um die Patientensicherheit zu erhöhen und interne Abläufe effizient zu gestalten, ist ein strukturiertes Qualitätsmanagement in Medizinischen Versorgungszentren ist essenziell. Die roXtra Dokumentenlenkung ermöglicht eine regelkonforme und übersichtliche Verwaltung Ihrer QM-Dokumentation – von Hygienekonzepten über Verfahrens- und Arbeitsanweisungen bis hin zu Notfallplänen. Mit unserer QM-Software lassen sich alle relevanten Dokumente im MVZ zentral erfassen, aktualisieren und verwalten.
Dank automatischer Versionierung, revisionssicherer Archivierung und anpassbarer Workflows behalten Sie jederzeit den Überblick über die Prüfung und Freigabe neuer oder geänderter Dokumente. Während eines Audits sind alle erforderlichen QM-Dokumente mit wichtigen Metadaten wie Revisionsstatus oder Wiedervorlagedatum sofort abrufbar.
Durch den Einsatz von roXtra wird das Qualitätsmanagement in Medizinischen Versorgungszentren optimiert, Fehlerquellen werden reduziert und eine vollständige Dokumentation sichergestellt. Eine leistungsstarke QM-Software im MVZ trägt dazu bei, gesetzliche Vorgaben einzuhalten, den Arbeitsalltag effizienter zu gestalten und Prozesse nachhaltig zu verbessern.
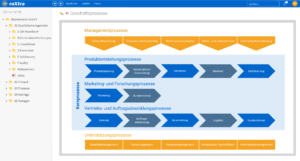
Prozesse digital steuern und optimieren
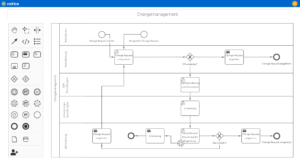
Ein gut strukturiertes und klar definiertes System ist die Basis für ein effektives Qualitätsmanagement in Medizinischen Versorgungszentren. Mit roXtra Prozesse lassen sich Prozesse digitalisieren und automatisieren, wodurch interne Abläufe – beispielsweise für Wartungspläne oder Urlaubsanträge – effizienter gestaltet werden. Durch die grafische Modellierung (BPMN 2.0) können Verantwortlichkeiten, Freigabeprozesse und Arbeitsabläufe flexibel angepasst und optimiert werden.
Der Flowchart-Designer ermöglicht eine schnelle Visualisierung von Flussdiagrammen, Organigrammen und Mindmaps, sodass Arbeitsabläufe strukturiert und verständlich dargestellt werden.
Der Flowchart-Designer ermöglicht eine schnelle Visualisierung von Flussdiagrammen, Organigrammen und Mindmaps, sodass Arbeitsabläufe strukturiert und verständlich dargestellt werden.
Mit roXtra Prozesse optimieren Sie Ihr Qualitätsmanagement in Medizinischen Versorgungszentren, automatisieren wiederkehrende Vorgänge und gewährleisten eine revisionssichere sowie normkonforme Dokumentation.
Strukturiertes Risikomanagement
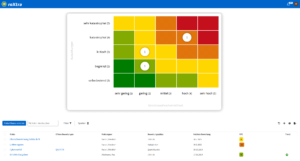
Ein umfassendes Qualitätsmanagement in Medizinischen Versorgungszentren beinhaltet auch ein strukturiertes Risikomanagement, um sowohl die Patientensicherheit als auch reibungslose Abläufe im MVZ zu gewährleisten. Mit roXtra können Sie potenzielle Risiken systematisch identifizieren, bewerten und verwalten, wodurch der Verwaltungsaufwand reduziert und eine gezielte Risikoanalyse ermöglicht wird. Die Software unterstützt etablierte Normen und Regularien wie ISO 9001, ISO 31000 und ISO 27001 sowie branchenspezifische Richtlinien, darunter die Rili-BÄK.
Regelmäßige Risikobewertungen sind entscheidend, um Gefahren frühzeitig zu erkennen und zu minimieren – sei es in den Bereichen Hygiene, Medizintechnik, Patientenversorgung oder Datenschutz. Ein strukturiertes Qualitätsmanagement für Medizinische Versorgungszentren trägt dazu bei, betriebliche Risiken zu reduzieren und eine sichere, normkonforme Organisation sicherzustellen.
Mit roXtra Risiken optimieren Sie Ihr Qualitätsmanagement in Medizinischen Versorgungszentren, stellen eine lückenlose Dokumentation sicher und minimieren Fehlerquellen. Eine leistungsstarke QM-Software für Ihr MVZ unterstützt Sie dabei, Risiken gezielt zu managen und eine transparente, revisionssichere Lösung für Audits und Zertifizierungen bereitzustellen.
Audits professionell steuern
Regelmäßige Audits sind ein wesentlicher Bestandteil, um die Einhaltung gesetzlicher Vorgaben und Qualitätsstandards sicherzustellen und ein strukturiertes Qualitätsmanagement in Medizinischen Versorgungszentren zu gewährleisten. Mit roXtra Audits optimieren Sie Ihr Auditmanagement und führen interne sowie externe Audits, beispielsweise nach ISO 19011, effizient und transparent durch.
Die QM-Software von roXtra ermöglicht einen standortunabhängigen Zugriff auf Auditfragenkataloge – auch offline. So behalten Sie jederzeit den Überblick über vergangene, aktuelle und geplante Audits und können Abweichungen sowie Optimierungspotenziale frühzeitig identifizieren. Sofortmaßnahmen, Korrektur- und Vorbeugemaßnahmen lassen sich direkt im Audit-Kontext dokumentieren und gezielt umsetzen.
Mit roXtra Audits wird das Qualitätsmanagement in Medizinischen Versorgungszentren normkonform, nachvollziehbar und effizient gestaltet. Die QM-Software unterstützt Sie dabei, Auditprozesse zu strukturieren und eine revisionssichere Dokumentation zu gewährleisten.
Maßnahmenmanagement für Medizinische Versorgungszentren
Ein effektives Qualitätsmanagement in Medizinischen Versorgungszentren setzt ein strukturiertes Maßnahmenmanagement voraus, um Abweichungen gezielt zu beheben, Risiken zu reduzieren und die Einhaltung gesetzlicher sowie normativer Anforderungen, wie ISO 9001, sicherzustellen. Mit roXtra Maßnahmen steuern Sie Sofort-, Korrektur- und Vorbeugemaßnahmen (CAPA) digital, systematisch und transparent.
roXtra Maßnahmen unterstützt eine lückenlose Dokumentation und strukturierte Überwachung aller Maßnahmen, sodass Sie kontinuierliche Verbesserungen gezielt umsetzen können.
Your advantages with roXtra measures:
- Central management: Clearly record and control all measures.
- Automatic effectiveness check: Sustainable implementation through planned checks.
- Simple evaluation: Filter lists of measures and export them as Excel, Word or PDF.
- Cross-module: Linking for consistent documentation.
Unterstützung für Qualitätsmanagement und Zertifizierungen für Praxen
Unsere Softwarelösungen für Medizinische Versorgungszentren unterstützen Sie sowohl im Alltag als auch bei der Zertifizierung und Re-Zertifizierung Ihres Qualitätsmanagementsystems. Mit roXtra setzen Sie eine Vielzahl von Normen und Regularien effizient um und gestalten das Qualitätsmanagement in Ihrem Medizinischen Versorgungszentrum sicher, transparent und regelkonform.
Quality management standards
- DIN EN ISO 9001 - Requirements for quality management systems
- DIN EN 15224 Quality management systems for the health care sector
- ISO 7101 Quality management systems in healthcare - Requirements
- DIN EN ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes
Risk management standards
- ISO 31000 - General guidelines on risk management
- ONR 49001 - Austrian standard for systematic risk management
- IEC 80001 standard for risk management in networked medical technology
Information security management
- ISO 27001 - Information Security Management System (ISMS) / IT Security
Specific guidelines and laws
- QEP (Quality and Development in Practices) of the Associations of Statutory Health Insurance Physicians (NASHIP) together with the Federal Association of Statutory Health Insurance Physicians
- KTQ Certification: Cooperation for Transparency and Quality in the Healthcare Sector
- Europäisches Praxisassessment (EPA)
- Claims of the Federal Joint Committee (G-BA), the German Accreditation Body GmbH (DAkkS), the German Nursing Council (Deutscher Pflegerat e.V. - DPR) as well as the Initiative Quality Medicine (IQM)
- KonTraG & Section 91 (2) AktG - Regulations on risk control in companies
One software, many application areas.
Warum ist Qualitätsmanagement in Medizinischen Versorgungszentren wichtig?
Um eine hohe Behandlungsqualität sicherzustellen, Patientensicherheit zu gewährleisten und gesetzliche Vorgaben wie ISO 9001 zu erfüllen, ist ein Qualitätsmanagement in Medizinischen Versorgungszentren essenziell. Zudem optimiert es interne Abläufe, reduziert Fehlerquellen und sorgt für eine effiziente Dokumentation sowie reibungslose Prozesse im MVZ.
Wie verbessert eine QM-Software das Qualitätsmanagement in einem MVZ?
Eine QM-Software verbessert das Qualitätsmanagement in Medizinischen Versorgungszentren, indem sie Prozesse automatisiert, Dokumente zentral verwaltet und Audits sowie Zertifizierungen erleichtert. Sie sorgt für transparente Abläufe, reduziert Fehlerquellen und unterstützt bei der Einhaltung gesetzlicher Vorgaben wie ISO 9001.
Wie unterstützt eine digitale QM-Software Medizinische Versorgungszentren bei Audits und Zertifizierungen?
Indem sie alle relevanten Dokumente zentral und revisionssicher verwaltet, Auditprozesse strukturiert und die Einhaltung von Normen wie ISO 9001 erleichtert, unterstützt eine Qualitätsmanagement-Software Medizinische Versorgungszentren bei Audits und Zertifizierungen. Sie ermöglicht eine lückenlose Nachverfolgung von Maßnahmen und sorgt für Transparenz sowie Effizienz im Auditmanagement.
roXtra supports you with the following solutions:
We will show you roXtra in a free and non-binding online presentation.






