The QM software for your laboratory
The QM software for your laboratory
Success Stories
Find out first-hand how laboratories use roXtra to make their laboratory quality management efficient, compliant and future-proof. Whether ISO 15189, GMP, GLP or comprehensive risk management - roXtra offers customized solutions for the specific requirements in laboratories.
Our blog provides you with valuable insights into accreditation and re-accreditation, the practical implementation of quality management in the laboratory as well as best practices and application examples from the industry.

Digital quality management at the laboratory service provider
TRIGA-S Scientific Solutions
Get advice now
Get to know roXtra in a non-binding and free online presentation.
What does quality management in the laboratory mean?
Quality management in the laboratory encompasses all measures, processes and systems that ensure that tests and analyses are carried out reliably, precisely and reproducibly. It is based on standards such as ISO 15189 for medical laboratories or ISO 17025 for testing and calibration laboratories and ensures that legal and regulatory requirements are met.
Why is QM important in the laboratory?
A well-implemented quality management system (QMS) improves the accuracy and reproducibility of laboratory results, minimizes sources of error and ensures efficient documentation. It contributes to the safety of patients and customers, optimizes work processes and increases the competitiveness of the laboratory. It also facilitates accreditations, audits and certifications, which strengthens the credibility and compliance of the laboratory.
In short, quality management in the laboratory is crucial to ensure high standards, reliability and efficiency - and thus to guarantee confidence in the results obtained.
What is the aim of a QM system according to ISO/IEC 17025?
ISO/IEC 17025 specifies the requirements for quality management systems in testing and calibration laboratories. Its central objective is to ensure technical competence and the reliability of results. A QM system in accordance with this standard is intended to ensure that laboratories apply validated procedures, take measurement uncertainties into account and maintain standard-compliant documentation.
The standard also promotes continuous improvement, transparency and compliance with legal and regulatory requirements. Structured quality management increases the trustworthiness of test and measurement results, which is essential for customers, authorities and accreditation bodies.
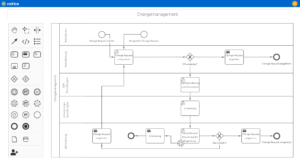
How do you record and analyze errors and deviations in the laboratory?
Errors and deviations in the laboratory must be systematically recorded, analyzed and corrected. QM software with action management facilitates this process by documenting deviations, analyzing causes and providing automatic workflows for corrective and preventive actions (CAPA).
Digital tools ensure transparent tracking, deadline control and complete documentation - essential for audits and certifications. Software-supported deviation management improves efficiency, compliance and quality assurance in the laboratory.
Which software or digital tools can improve quality management in the laboratory?
Modern software solutions facilitate quality management in the laboratory by automating processes, simplifying documentation and ensuring compliance with standards such as ISO 15189 or ISO/IEC 17025. Document management systems (DMS) support the versioning and approval of SOPs, while action management tools record deviations, analyze causes and control CAPA processes.
In addition, laboratory information and management systems (LIMS) improve the traceability of samples and results. Audit and training software helps to document evidence and meet certification requirements. The digitalization of quality management significantly increases efficiency, traceability and compliance in the laboratory.
One software, many application areas.
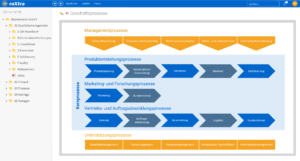
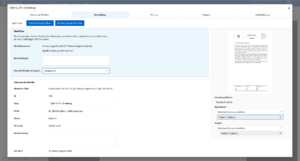
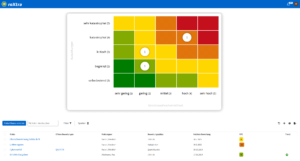
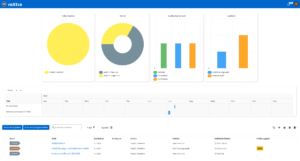
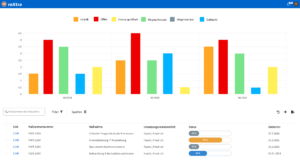
Efficient quality management
With roXtra Documents you can manage your QM documentation in the laboratory - from test procedures and service specifications to hygiene management and SOPs - effortlessly and in compliance with regulations. Our document management system (DMS) supports the entire document lifecycle in accordance with ISO/IEC 17025 and other relevant standards.
Thanks to automatic versioning, audit-proof archiving and individual workflows, you can organize the review and approval of new and revised work and process instructions efficiently and transparently. During an audit, you can provide auditors with an overview of all relevant QM documents including metadata (e.g. resubmission date, status, revision) with just a few clicks.
With roXtra, you can optimize quality management in the laboratory, minimize sources of error and ensure complete traceability - digitally, securely and in compliance with standards.
roXtra supports you with the following solutions
We will show you roXtra in a free and non-binding online presentation.