The QM software for the
healthcare sector
The QM solution for the healthcare sector
Success Stories
Find out first-hand how our customers use roXtra to make their quality management efficient, compliant and future-proof. Whether ISO 9001, KTQ or comprehensive risk management - roXtra offers customized solutions for the specific requirements of QM software in the healthcare sector.
Our blog provides you with valuable insights into accreditation and re-accreditation, the practical implementation of quality management in clinics as well as best practices and application examples from the industry.
Get advice now
Get to know roXtra in a non-binding and free online presentation.
One software, many application areas.
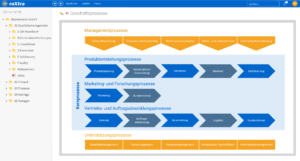
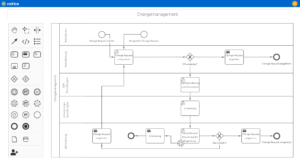
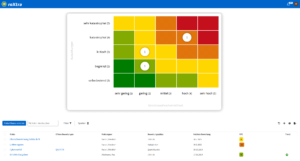
Support for quality management and certification in clinics
Our software solutions support you in daily system operation as well as during the certification and re-certification of your quality management system. With roXtra, implementing a large number of standards and regulations becomes a breeze.
Quality management standards
- DIN EN ISO 9000 and DIN EN ISO 9001 Quality management systems - Requirements
- KTQ certification: Cooperation for transparency and quality in healthcare
- DIN EN 15224 Quality management systems for healthcare
- ISO 7101 Quality management systems in healthcare - Requirements
- DIN EN ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes
- NÖ BASIS ZERT
Risk management standards
- DIN EN ISO 31000 - Guidelines for risk management
- ONR 49000 / ONR 49001 - Austrian risk management standard
- IEC 80001 standard for risk management in networked medical technology
- § Section 91 (2) AktG - Establishment of a risk management system (RMS) for listed companies
Information security management
- ISO 27001 - Information security management system (ISMS) / IT security
Specific guidelines and laws
- Requirements for operators of critical infrastructures (KRITIS)
- Claims of the Joint Federal Committee (G-BA), the German Hospital Federation (DKG), the German Accreditation Body (DAkkS), the German Nursing Council (DPR) and the Quality Medicine Initiative (IQM)
- KonTraG - Law on Control and Transparency in the Corporate Sector (Corporate Governance)
roXtra supports you with the following solutions
Quality management software in hospitals
A hospital is a healthcare center where patients trust that all processes will run smoothly and that medical care will be provided optimally. In order to guarantee this patient orientation and safety as well as a high quality of treatment, reliable quality management (QM) tailored to internal hospital processes is essential. QM in the healthcare sector is an ongoing process that requires systematic and continuous evaluation. It serves to improve the quality of products and services and helps to achieve better results.
The solution: roXtra as QM software for clinics.
We configure the system specifically to your requirements, allowing you to implement your corporate design as well as regulatory and technical requirements. Maintain an overview thanks to your individual folder structure or display the documents according to standard structures (e.g. KTQ, ISO 9001, ISO 7101, DIN EN 15224, Onkozert and DIN 6870) at the click of a mouse. The QM software for clinics also meets the requirements of the GxP-regulated environment. 🗎Here you will find an overview of how the criteria of the EU-EMP guidelines (Annex 11) and the guidelines of the German Medical Association (Rili-BÄK), for example, are implemented. The focus here is clearly on patient safety. We are also happy to support you with implementation, validation, certification and throughout the entire system operation.
We will show you roXtra in a free and non-binding online presentation.









